Research
Research Overview: Studying and Targeting Advanced Lung Cancer
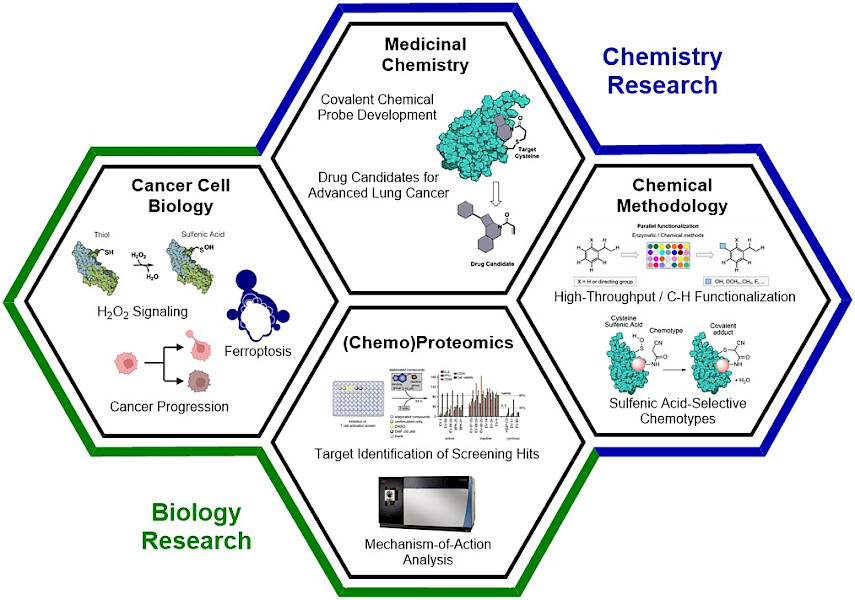
The Konrad laboratory has established an interdisciplinary research program that encompasses chemistry and biology-oriented segments, which are highly interconnected. Our key goal is the development of covalent chemical probes and drug candidates that specifically target advanced non-small cell lung cancer. Lung cancer is the leading cause of cancer-related mortality worldwide and 85% of all cases are Non-Small Cell Lung Cancer (NSCLC). The death toll of lung cancer is a result the high adaptability and evasiveness of NSCLC which leads to the development of therapy-resistance when treated. This is complemented by a late discovery of the disease, where the advanced cancer has metastasized and is resistant to currently available therapeutics. As such, it is important to develop new therapeutic approaches that specifically target advanced lung cancers. To identify small molecules that engage proteins which constitute vulnerabilities ("weaknesses") in these cancers, we are using (chemo)proteomics analyses. These small molecules are then used as chemical probes to study the underlying biological mechanism and as lead structures for medicinal chemistry. Since medicinal chemistry studies are extensively time-consuming endeavors, it is a key focus of our research to accelerate structure-activity relationship studies and be able to quickly access structures with high potencies and low off-target engagement. For this purpose, we are working on chemical methodologies for late-stage functionalizations that harness high-throughput experimentation and machine learning.
Uncovering and Harnessing New Vulnerabilities for the Treatment oder Advanced Cancers
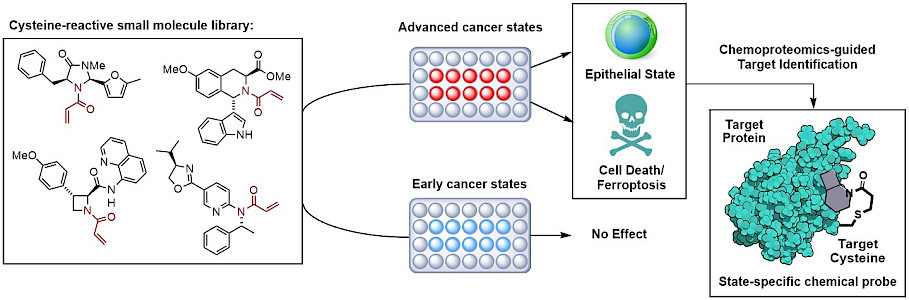
A focus of our research is establishing chemical methodologies that allow the synthesis and modification of new chiral drug scaffolds. These scaffolds are employed in covalent fragment-based screenings that combine phenotypic analysis in NSCLCs with the mass spectrometry-based chemical proteomics method Activity-Based Protein Profiling (ABPP). ABPP, in general, allows the analysis of a reaction between a chemical probe and a whole proteome. This approach enables the expedient identification of the protein targets of our small molecule scaffolds that lead to the observed phenotype in NSCLCs. The compounds serve as chemical tools to uncover the mechanisms as well as pathways that are associated with their target proteins and can be advanced into drug candidates by using medicinal chemistry. It is worth noting that the great potential of ABPP includes furnishing global structure-activity relationships (SARs), i.e. for all detectable proteins within the evaluated proteomes in parallel. This feature allows to assess and optimize multiple parameters of the drug candidates, such as the affinity for the desired protein as well as for the off-targets, simultaneously. In addition, if substitution patterns that are incorporated into the scaffolds do not benefit the affinity to the target binding pocket, ABPP will provide insights into which proteins are susceptible to the evaluated substituents. Our approach to develop a broad library of drugs candidates is motivated by the diversity of NSCLC genotypes. The availability of an arsenal of drugs that target different cancer pathways is a first step towards personalized and precise cancer treatments.
Inducing Ferroptotic Cell Death in Lung Cancers
Advanced drugs that are approved for the treatment of NSCLC, such as the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) stall cancer progression by blocking angiogenesis and migration. Since the cancer cells remain alive, however, they can gradually develop resistances to the inhibitors. Therefore, it would be highly desirable to develop pharmaceutical agents that directly induce cell death to circumvent this adaptive mechanism. In contrast to induction of apopotosis, which is frequently suppressed in cancers, a cell death mechanism that remains active in many cases and thus creates a vulnerability is the ferroptotic pathway. To harness the induction of ferroptosis towards cancer treatment, we are developing chemical probes that allow us to study mechanisms that are associated with this unusual oxidative cell death pathway and use medicinal chemistry to advance these tools into drug candidates.
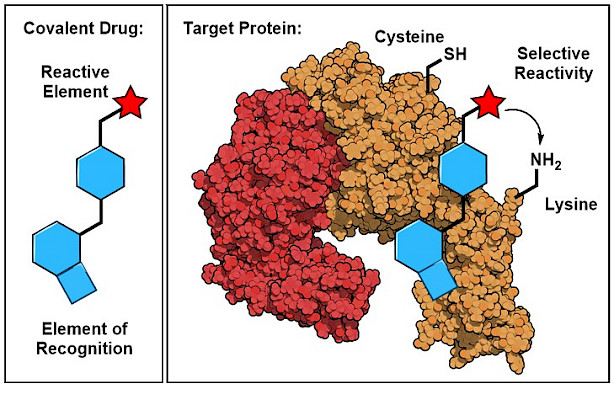
Chemical Reactions for Therapeutic Interventions
The site-selective functionalization of amino acid residues on a native protein within the human proteome is one of the most significant challenges in organic chemistry and chemical biology. This challenge is met in the development of reactivity-based therapeutics, such as covalent inhibitors. Covalent inhibitors contain carefully designed ligand structures that reversibly bind to a targeted protein site. In contrast to traditional drugs, they are additionally equipped with an electrophilic functionality (named ‘chemotype’ or ‘warhead’) that reacts with a defined amino acid residue in proximity to the binding site and thereby irreversibly modifies the protein-of-interest. To date, only a small set of reliable, stable and selective chemotypes are available. A prominent example is acrylamide, which selectively reacts with the highly nucleophilic cysteine and selenocysteine residues. Based on this discovery, a new class of covalent anticancer drugs has emerged which recently entered the pharmaceutical market. Afatinib, for instance, is a covalent tyrosine kinase inhibitor which is used for the treatment of NSCLC. Our research aims to design electrophiles that enable reliable and selective reactions with specific amino acids to form stable covalent bonds as basis for the development of new classes of reactivity-based therapeutics.